Selection includes high selectivity and low detection limit sensors
WPI's biosensors are unique, because they offer a high selectivity and low detection limit (down to nM concentration) with a broad dynamic range, covering physiological concentrations of species with different sizes from nanometer to millimeter. The majority of our sensors are the only commercially available sensors in the world. Scientists across a variety of disciplines have relied on our sensors for over 25 years. These scientists use WPI's sensors for research performed in universities, hospitals, biomedical research labs, pharmaceutical companies, food/nutrition research labs, environmental monitoring centers and military labs. Our popular biosensors are listed in thousands of publications.
- Carbon Monoxide Sensors :
Carbon monoxide (CO) is a versatile mediator of physiological processes. Carbon monoxide (CO) formed by internal mechanisms (endogenous) is measured in a variety of ways, but standard measurement methods are of limited utility in most biological systems. WPI's ingenious ISO-COP-2 CO sensor measures CO in vivo or in vitro in real time! Our carbon monoxide sensors work with the TBR4100 and TBR1025 free radical analyzers. - Glucose Sensors :
Measuring glucose in vivo over the long term is challenging and difficult. Previous measurement systems were limited to acute studies or a few days at best. WPI introduces a new kind of implantable glucose sensor based on a patented technology. This sensor provides a tool for researchers to directly detect glucose in chronic studies in vitro or in vivo. The sensor is fully compatible with WPI's Apollo system. Our glucose sensors work with the TBR4100 and TBR1025 free radical analyzers. - Hydrogen Peroxide Sensors :
Hydrogen Peroxide is produced in biological systems by controlled pathways at low concentrations that impact on cell signaling. At higher concentrations inflammatory cells produce local intense amounts of this oxidant to kill pathogens. In the progress of human disease, uncontrolled formation of hydrogen peroxide from the mitochondrial respiratory chain and enzymes, such as xanthine oxidase, can occur (Prof. Victor Darley-Usmar, Univ. of Alabama, personal communication). Despite the recognized importance of this oxidant in biology real-time measurements at low concentration have been difficult. The hydrogen peroxide sensors developed by WPI are designed to compliment existing high sensitivity fluorescent approaches with direct quantitative measurement in biological samples in the low nM range. Four hydrogen peroxide sensors are currently available. The ISO-HPO-2 is a 2.0 mm stainless steel sensor, with replaceable membrane sleeves (#600012) and an internal refillable electrolyte (#100042). The sensor is designed for use in cell cultures and similar applications. The ISO-HPO-100 is a 100 micron tip diameter hydrogen peroxide micro sensor designed for use in tissues and similar applications. The sensor design is based on a flexible “activated” carbon fiber sensing electrode coated with a proprietary membrane that enhances hydrogen peroxide detection. Our hydrogen peroxide (H2O2) sensors work with the TBR4100 and TBR1025 free radical analyzers. - Hydrogen Sulfide Sensors :
Although hydrogen sulfide (H2S) is generally thought of in terms of a poisonous gas, it is endogenously produced in many mammalian tissues. It has been detected in micromolar amounts in blood and brain tissue. Hydrogen sulfide is reported as having a broad range of biological functions and although its potential to participate in cell signaling is clear, this biological role is not well understood. H2S is strongly analogous to nitric oxide (NO), because they share several physical and metabolic properties. Like NO, H2S is a potent vascular signal that can mediate vasoconstriction or vasorelaxation depending on the O2 level and tissue. In the rat aorta, H2S concentrations that mediate rapid constriction at one O2 level will cause rapid relaxation at lower O2 levels. The ISO-H2S-2 sensor is a low detection limit sensor that works with WPI'sTBR4100 and TBR1025 free radical analyzers to record H2S in vitro. It is the only sensor available that measures H2S. - Nitric Oxide Sensors :
WPI offers the most extensive range of nitric oxide (NO) sensors available. Developed over a decade of extensive research in the field of nitric oxide, the result is a superior range of NO sensors that enable routine detection of nitric oxide at ultra low concentrations. Selectivity of WPI's Nitric Oxide Sensors The ideal nitric oxide sensor should be insensitive to other reactive species likely to be present within the measurement environment. The conventional Nafion coated carbon fiber nitric oxide sensor exhibits a large response to such species. WPI’s unique nitric oxide sensor technology utilizes a novel surface membrane which amplifies the response to NO while eliminating responses to a vast range of reactive species, including nitrite, absorbic acid, hydrogen peroxide, catecolamines, and much more. Nitric Oxide Sensor Guide Applications In Vivo Cell Cultures, NO2 NO3 Tissue Bath Microvessels Microvessels Single Cell Sensor ISO-NOPF ISO-NOP ISO-NOP30L ISO-NOP30 ISO-NOP007 ISO-NOPNM Sensor Diameter 100, 200 or 500µm 2mm 30µm 30µm 7µm 100nm Response Time < 5 sec < 5 sec < 3 sec < 3 sec < 3 sec < 3 sec Lowest Detection Limit 0.2 nM 1 nM 1 nM 1 nM 0.5 nM 0.5 nM Temperature Sensitivity some yes yes yes yes some Drift none none none none none none Sensitivity 10 pA/nM 2 pA/nM 1.4 pA/nM 1.4 pA/nM 1 pA/nM 0.5 pA/nM Physiological Interferent none none none none none none Our nitric oxide (NO) sensors work with the TBR4100 and TBR1025 free radical analyzers.
Comparing Sensors
Choosing the right sensor for your application is critical for successful research. The best way to determine compatibility is to test a sensor in your application, however, cost can make that impossible.Therefore, you must rely on the specifications and information provided by the manufacturer. Five performance factors help you make an informed choice for your given application :
Response
Electrochemical electrodes produce changes in current in response to changes in concentration. "Response" is most often specified in terms of the amount of current per concentration unit: nA/micromole or pA/nM, etc. The larger the current per unit the higher the sensitivity of the sensor.
Detection Limit
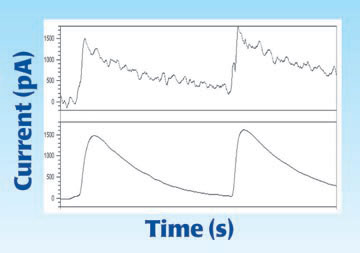
The response of a given free radical sensor is meaningless without also specifying the detection limit. Detection limit is the minimum change in concentration that can be reliably seen. This specification is directly related to the noise of the sensor. A sensor with a 100nA/μM response but a 3μM detection limit is not as good as a 10nA/μM response sensor with a 1μM detection limit.
The best sensors have low detection limits and high sensitivity. In the graph above, the response to the addition of 50nM of nitric oxide is shown for the WPI flexible NO sensor and the comparable Brand Z electrodes. The measurements were made with the same meter simultaneously. As can be seen from the graphs response is similar but the noise level on the WPI electrode allows for a more precise estimate of the current.
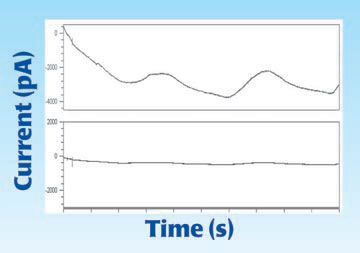
Drift
A sensor can have a low detection limit and a good response, however, to be useful in long term studies it must be stable when temperature and concentration are constant. A drifting baseline, if monotonic, can be corrected, but wandering baselines limit the utility of sensors to short experiments.
Selectivity
It is a rare instance that the ion species of interest is the only ion in the medium to be measured. In a perfect world your sensor would respond ONLY to the ion of interest. In reality there is always some contribution from competing species. The lower the contribution the better. Graphs detailing the impact of competing species on electrode output are shown on the performance page of this brochure.
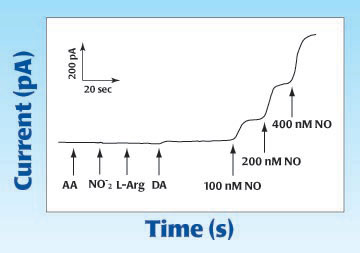
The first graph below shows the response of WPI's ISO-NOP007 nitric oxide microsensor following additions of: 50μM ascorbic acid (AA), 50μM nitrite, 100μM L-Arginine (L-Arg), 20 μM Dopamine (DA), 100 nM and 200nM NO. The graph indicates no interference to common reactive species, plus an enhanced response to NO. [Zhang, et al., 2000.]
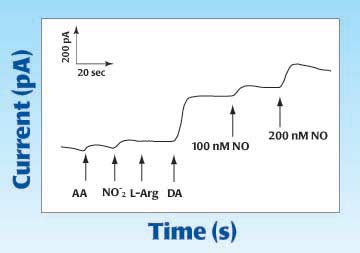
The second graph below shows the response of typical Nafion-coated nitric oxide carbon microelectrode following additions of: 50μM ascorbic acid (AA), 50μM nitrite, 100μML-Arginine (L-Arg), 2μM Dopamine (DA), 100 nM and 200nM NO. [Zhang,et al., 2000.]
Linearity
For an electrode to be useful and easy to calibrate the response must be "Linear" with changes in concentration over the range of interest. Non-linear behavior requires special curve fit software to calibrate the sensors. This approach is more time consuming and can be unreliable. "Good" linearity is expressed by a R2 of 0.900 or higher. (1.00 is perfect.) All of the electrochemical sensors made by WPI are have a 0.9 or better.

